General description of Bromoethane
General description
Bromoethane is a bromoalkane that is ethane carrying a bromo substituent. It is an alkylating agent used as a chemical intermediate in various organic syntheses. It has a role as a carcinogenic agent, a solvent, a refrigerant, a local anaesthetic and an alkylating agent. It is a bromoalkane, a bromohydrocarbon and a volatile organic compound. Ethyl bromide appears as a colorless volatile liquid. Slightly soluble in water and denser than water. Flash point below 0°F. Vapors are heavier than air. Toxic by inhalation. Irritates skin and eyes. Used to make pharmaceuticals and as a solvent. Bromoethane, also known as ethyl bromide, is a halogenated hydrocarbon with the chemical formula of C2H5Br and the abbreviation of EtBr. It is a colorless oily liquid. It has an ether like smell and burning smell. It gradually turns yellow when exposed to air or light. It is volatile and can be miscible with ethanol, ether, chloroform and most organic solvents.
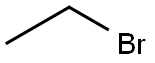
Figure 1 The chemical structure of Bromoethane
Application and Pharmacology
It is an important raw material for organic synthesis. In agriculture, it is used as fumigating insecticide for grain storage, warehouses and houses. Bromoethane is formed by the reaction of potassium bromide with frozen sulfuric acid and ethanol. Commonly used in the ethylation of gasoline, refrigerant and anesthetic. Therefore, chemical production workers and fumigants can be exposed to different concentrations of bromoethane. Bromoethane is the raw material for barbital production. It is also used as refrigerant, anesthetic, solvent, fumigant, organic synthesis, etc. Used as analytical reagent, such as solvent and refractive index standard sample. It is also used as fire extinguishing agent for organic synthesis and aviation industry. It is used in organic synthesis, solvent, refrigerant and pharmaceutical industry[1].
Synthesis
1.It is obtained by the reaction of ethanol and sodium bromide: add 40% sodium bromide solution and ethanol into the reaction pot, add sulfuric acid under stirring, and the temperature shall not exceed 50 ℃. After addition, the reaction shall be carried out at 45-50 ℃ for 2h. Distillation, the distillate is neutralized with 3% sodium carbonate solution, and the organic phase is bromoethane after standing for 2h. Raw material consumption quota: 557kg/t ethanol (95%), 1610kg/t hydrobromic acid (48%), 1165kg/t sulfuric acid (92%).
2. it is obtained by the reaction of ethanol and bromine: bromine is slowly added to the suspension of sulfur and water under stirring, and the temperature is maintained at 35-40 ℃. Then add ethanol. Add sulfuric acid at 25-28 ℃ and react for 42h. Distill and collect the 38-39 ℃ fraction to obtain bromoethane. The yield is about 90%. Raw material consumption quota: 770kg/t ethanol, 250kg/t bromine, 1042kg/t sulfuric acid and 18kg/t sulfur. In method (1), hydrobromic acid can also be used to react with ethanol. The preparation process is the same as that of sodium bromide method, and the yield is 96%. In addition, bromoethane can be obtained from the addition of ethylene and hydrogen bromide or the bromination of ethane. Purity of industrial bromoethane ≥ 98%.
3. take the industrial product bromoethane as raw material, wash it with concentrated sulfuric acid for several times, wash it with water once, wash it with 10% sodium carbonate solution twice, and wash it with 5% sodium thiosulfate solution once, leave it still to remove the water layer, dry it with anhydrous calcium chloride, distill it, and collect the 37 ~ 40 ℃ fraction as the finished product.
4. preparation method: add 41.5g (2.81mol) of 48% hydrobromic acid to the reaction bottle equipped with stirrer, dropping funnel and distillation device, cool it in water bath, slowly add 120g (65ml) of concentrated sulfuric acid under stirring, then add 100g (2.06mol) of 95% ethanol (2), slowly heat it, drop 109ml of concentrated sulfuric acid from the dropping funnel, and evaporate the generated bromoethane at the same time. The connecting pipe extends under the water in the receiving bottle, and the receiving bottle is cooled with ice water. Stop the reaction when no bromoethane is evaporated. The crude bromoethane of the fractionated species is fractionated, washed with water, 5% sodium carbonate solution and water, and dried with anhydrous calcium chloride. The distillates at 38 ~ 39 ℃ were collected by water bath heating and distillation to obtain 205g bromoethane (1) in 91% yield.
5. preparation method: add 100ml (1.7mol) of 95% ethanol (2) and 90ml of water into the reaction bottle, slowly add 190ml of concentrated sulfuric acid under continuous stirring, the reaction is exothermic, and pay attention to cooling to room temperature. Slowly add 163g (1.5ml) of finely ground sodium bromide and several zeolites under stirring. Install the distillation unit, add an appropriate amount of ice water into the receiving bottle, and place it in the ice bath. Heat slowly to make the reaction and distillation proceed smoothly until no oil is distilled out. Separate the organic layer and dry it with sulfuric acid. Distill and collect the distillates at 35 ~ 40 ℃ to obtain 100 ~ 120g of bromoethane (1), with a yield of 61% ~ 74%.
6. The antimicrobial action of partially quaternized poly(2-(dimethylamino)ethyl methacrylate) (PQDMAEMA) copolymers using different alkyl halides is presented. The poly(2 (dimethylamino)ethyl methacrylate) (PDMAEMA) homopolymer was synthesized by group transfer polymerization, followed by the modification of its tertiary amine groups, using bromoethane, iodoethane, bromohexane and bromoethanol, to introduce permanent cationic, quaternary ammoniumsalt moieties, randomly distributed along the polymer chains. In all cases, the degree of quarter nization was low, at ~10 mol%, as verified by proton nuclear magnetic resonance spectroscopy to preserve the thermo-responsive character of the PDMAEMA precursor polymer. The biocidal activity of the lightly quaternized PQDMAEMA copolymers against Escherichia coli and Staphylococcus aureus was evaluated by calculating the minimum inhibitory concentration (MIC) as well as the minimum bactericidal concentration (MBC) of the polymers and by comparing them to the respective values of the precursor non-quaternized PDMAEMA homopolymer. The antibacterial mechanism of action in the solution was studied by zeta potential measurements, scanning electron microscopy and protein leakage tests signifying the disruption of the outer membrane of the bacterial cells to release their periplasmic proteins[2].
Safety
At first, eugenol was used as a perfume. Later, it was found to have insect repellent and antibacterial effects, as well as its good anti-tumor activity, indicating that eugenol has a great development prospect. In terms of pharmacological activity, anti-tumor effect of eugenol can not be ignored. MF et al. found that eugenol has anti proliferation effect on human cervical cancer cells, which is mainly achieved by inducing apoptosis. ISS et al. [6] found that eugenol was added to the anticancer drug cisplatin. They found that eugenol could enhance the inhibitory effect of cisplatin on breast cancer stem cells by inhibiting ALDH enzyme activity. At the same time, the study showed that eugenol enhanced the effect of cisplatin on NF- κ Inhibition of B signal pathway[3].
Reference
1. Kanaparthi, D., et al., Methane emission from feather moss stands. Glob Chang Biol, 2017. 23(11): p. 4884-4895.
2. Manouras, T., et al., Responsive Quaternized PDMAEMA Copolymers with Antimicrobial Action. Polymers (Basel), 2021. 13(18).
3. Schuler, P., et al., One-Step Synthesis and Schlenk-Type Equilibrium of Cyclopentadienylmagnesium Bromides. Chemistry, 2021. 27(62): p. 15508-15515.
Lastest Price from Bromoethane manufacturers

US $0.00/KG2025-12-02
- CAS:
- 74-96-4
- Min. Order:
- 1KG
- Purity:
- ≥99%
- Supply Ability:
- 80 Tons/Month

US $4200.00/T2024-12-13
- CAS:
- 74-96-4
- Min. Order:
- 1KG
- Purity:
- 99% Min.
- Supply Ability:
- 300 tons per month